electronic configuration of iron in shells|Electron configuration of iron : Tuguegarao In order to write the Calcium electron configuration we first need to know the . Watch Futanari Teacher and Futanari Schoolgirl video on xHamster, the largest sex tube site with tons of free Japanese Hermaphrodite & Teacher porn movies!All over the world, local libraries offer millions of ebooks and audiobooks. You can borrow them — for free, instantly — with a library card and Libby: the award-winning, much-loved app for libraries.
PH0 · Orbital configuration for iron
PH1 · Electronic Configuration of Iron: Fe element
PH2 · Electronic Configuration of Iron
PH3 · Electron configuration of iron
PH4 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH5 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH6 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH9 · 3.4: Electronic Structure of Atoms (Electron Configurations)
By clicking “Proceed”, I hereby give consent to the Philippine Statistics Authority (PSA) to use my demographic information (i.e.First Name, Middle Name (optional), Last Name, and Date of Birth) and Facial Image, as submitted through the National ID website, to verify my identity and retrieve my digital National ID.
electronic configuration of iron in shells*******Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the .
In order to write the Calcium electron configuration we first need to know the .electronic configuration of iron in shells Electron configuration of iron Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .electronic configuration of iron in shellsHow to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since .Since 1s can only hold two electrons the next 2 electrons for magnesium go in .
Chlorine Electron Configuration - Electron Configuration for Iron (Fe, Fe2+, and .Sulfur Electron Configuration - Electron Configuration for Iron (Fe, Fe2+, and .
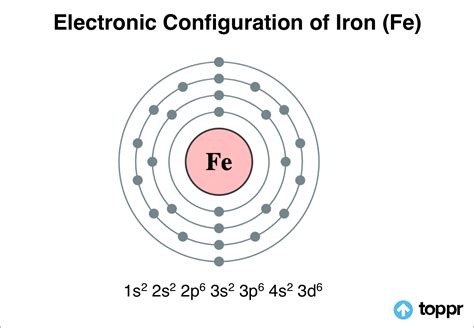
Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .Electronic configuration of iron is [Ar] 3d64s2. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to . Mar 23, 2023 The electronic configuration of Iron is a symbolic depiction of how its atoms' electrons are distributed in different atomic orbital atoms. Electronic Configuration of Iron is - 1s2 2s2 . The main shell electronic configuration is 2,8,8,8. According to the definition, the outermost number of electrons is 8 so the valency may be 8. But experimentally .
Fe, or iron, is a transition metal with atomic number 26. It has a unique electron configuration due to its location in the Periodic Table. The orbital diagram for Fe can be .
Fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and .
Electronic Configuration of Iron is is: 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Iron is a substance component with a nuclear number 26 find out about Iron and uses of the Iron .The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three . Example \(\PageIndex{1}\): Iron. Consider the electronic structure of neutral iron and iron (III). To write the electronic structure for Fe 3 +: Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2; Fe 3 +: 1s 2 2s 2 2p 6 .
Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere. Electron configurations describe where electrons are located around the nucleus of an atom. . Electron shells are divided into subshells, which are further divided into orbitals. . Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Full Electron Configuration Electron shell arrangement; 1: Electron configuration of Hydrogen (H) 1s 1: 1s 1: 1: 2: . Electron configuration of Iron (Fe) [Ar] 3d 6 4s 2: 1s 2 .For example, all the alkali metals have "isoelectronic" valence shell electron configurations. That means their outer shells have similar numbers of electrons in similar orbitals, which for the alkali metals have a ns 1 electron configuration, where n is the outer (valence) shell. Because of this they behave similarly, and for example, can .Examples of some neutral atoms and their electron configurations are shown below. In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Similarly, neon has a complete outer 2n shell containing eight electrons. These electron configurations make helium and neon very stable. Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 3.4.6 3.4. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:Electron configuration of iron For example in K shells almost $ 2 $ electrons can be there. Through some experiments it is found that in the valence shell almost $ 8 $ electrons can be there. So if Iron $ \left( {Fe} \right) $ electronic configuration would be like $ 2,8,16 $ , then we can see that the valence shell would not be a stable one.Electron Configuration of Oxygen. The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order: K shell – 2 electrons. L shell – 6 electrons. Therefore, the electron configuration of oxygen is 1s 2 2s 2 2p 4, as shown in the illustration provided below. Chlorine Electronic .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .

Hence, the 3d orbitals are occupied (and NOT the 4p !), as expected by the Aufbau principle (iron is not some weird exception here!). The 3d orbitals are in the so-called " M shell", so in " KLM " notation (which is not particularly clear, but is nevertheless used for X-ray experiments), one would write 2,8,14,2.Electron shell. In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom 's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the .
The number of electrons in the valence shell of iron (Fe) can be used to calculate the electronic configuration of the various oxidation states. Fe2+ F e 2 +: Iron now has an electron configuration of [Ar]3d6 [ A r] 3 d 6 after losing two electrons from its 4s orbital. The iron ion acquires a 2+ charge as a result. Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,.. For .Electron configurations of the elements (data page) This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the .
The main shell electronic configuration is 2,8,8,8. According to the definition, the outermost number of electrons is 8 so the valency may be 8. . The electronic configuration of iron will be reduced to [Ar]3d6. And the Oxidation state of iron becomes +2. Iron will attain more stability by the loss of one more electron from the d . Basic Steps. Writing an electron configuration for a transition metal element follows the same basic steps as for writing an electron configuration for an element in the s-block or p-block. List each subshell, and then fill each subshell with an appropriate number of electrons until all electrons in the element are accounted for.
Iron -. Fe: properties of free atoms. Iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is [ Ar ]. 3d6. 4s2 and the term symbol is 5D4. Schematic electronic configuration of iron. The Kossel shell structure of iron.
Amazon.ca: motorcycle tires. Skip to main content.ca. Delivering to Balzac T4B 2T Update location All. Select the department you . Dunlop D404 Rear Motorcycle Tire 150/80B-16 (71H) Black Wall. 4.7 out of 5 stars. 1,091. $188.31 $ 188. 31. FREE delivery Jul 11 - 15 . Only 1 left in stock.
electronic configuration of iron in shells|Electron configuration of iron